Explain the procedure of finding Specific heat of a solid experimentally.
(or)
Determine the experment to find out the Specific heat of a solid.
Answer :
Aim : To find out the Specific Heat of a solid experimentally.
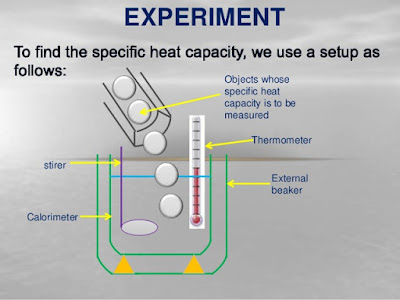
Apparatus : Calorimeter, Thermometer, Stirrer, Water, Steam Heater, Wooden box and Lead Shots.
Procedure :
- Measure the mass of calorimeter with stirrer = m1 gm
- Fill the water in Calorimeter and measure the mass = m2 gm and mark it's temperature as T1.
- Mass of the water = (m2 - m1) gm.
- Take few lead shots in steam heater and heat up to 100℃. Let this temperature be T2.
- Pour lead shots into calorimeter and measure the final temperature T3.
- Mass of Calorimeter with Water and Leadshots is m3 gm. Mass of lead shots = (m3 - m2) gm.
- Consider the Specific Heats of Calorimeter, Lead shots and water be Sc, Sl and Sw respectively.
- We know that Heat lost by hot body = Heat gained by cold bodies.
(m3 - m1)Sl(T2 - T3) = m1Sc(T3 - T1) + (m2 - m1)Sw(T3 - T1) - Knowing the specific heats of calorimeter and water, we can calculate the Specific heat of given solid.


nice
ReplyDelete